Author guidelines
Type of articles
The Weekly Epidemiological Surveillance Report (WESR) classifies published articles into five main categories.
- Original articles
- Outbreak investigation
- Public health program evaluation and surveillance evaluation
- Epidemiological study
- Case report
- Disease prevention and control-related research study
- Weekly summary report of disease outbreaks/health threat verifications
- Summary report of epidemiological surveillance activities
- Disease/Health Threat Situational Report
- Other Article Types: Editorial Note/Note from the field/Health Alert Reports/Guidelines for disease surveillance, investigation and control/Review article
WESR is published on a basis comprising 2-3 original articles, weekly summary report of disease outbreak/health threat verifications published 4-5 articles, summary report of epidemiological surveillance activities published 2-3 articles, disease/health threat situational report published 1-2 articles, and other article types are available on a case-by-case basis.
Types of articles published in WESR
1. Original articles
1.1 Epidemiological Investigations:
Reports of outbreak or public health threat investigations, including causative factors, sources, transmission, and patterns of spread.
Structure: Abstract, Keywords, Introduction, Methods, Ethical Considerations, Results, Discussion, Study Limitations, Control and Prevention Measures, Recommendations, Conclusion, Acknowledgements, Funding, Conflicts of Interest, AI Declaration, References.
Length: <14 pages; <4 tables/figures/boxes; >10 references.
1.2 Public Health Program or Surveillance Evaluation: Assessment of public health programs or surveillance systems related to communicable, noncommunicable, and occupational diseases.
Structure: Abstract, Keywords, Introduction, Methods, Ethical Considerations, Results, Discussion, Study Limitations, Recommendations, Conclusion, Acknowledgements, Funding, Conflicts of Interest, AI Declaration, References.
Length: <14 pages; <4 tables/figures/boxes; >10 references.
1.3 Epidemiological Studies:
Analysis of surveillance or statistical data at national or regional levels, describing person, place, and time distribution, with recommendations or policy implications.
Structure: Abstract, Keywords, Introduction, Methods, Ethical Considerations, Results, Discussion, Study Limitations, Recommendations, Conclusion, Acknowledgements, Funding, Conflicts of Interest, AI Declaration, References.
Length: <14 pages; <4 tables/figures/boxes; >10 references.
1.4 Case Reports: Detailed reports of rare or novel cases or syndromes.
Structure: Case background, Patient information, Clinical notes, Case description, Clinical course, Case summary, Discussion/Observations, Informed Consent, References.
Length: <10 pages; <4 tables/figures; >10 references.
1.5 Research Studies in Disease Prevention and Control:
Research addressing questions related to disease prevention, control measures, policy, or law, with ethical considerations.
Structure: Abstract, Introduction, Methods (including materials), Ethical Considerations, Results, Discussion, Study Limitations, Conclusion, Recommendations, Acknowledgements, Funding, Conflicts of Interest, AI Declaration, References.
Length: <14 pages; <5 tables/figures/boxes; >10 references.
2. Weekly Outbreak Verification Reports
Prepared by the WATCH team, summarizing weekly outbreak events from provincial health offices, regional disease control centers, Bangkok Metropolitan Administration, and other relevant agencies.
Content: Outbreak investigation details, risk factors, prevention and control measures taken or required, risk assessment, international outbreak reports (optional), supporting tables/figures (optional).
Length: <10 pages.
3. Epidemiological Surveillance Reports
Compiled by the Bureau of Epidemiology, presenting national-level patient and mortality data from notifiable disease surveillance systems.
Content: Case counts and deaths by area, trends, reporting coverage, and summary tables.




4. Disease/Health Threat Situation Reports
Prepared by staff of the Bureau of Epidemiology or Department of Disease Control, summarizing national or international disease situations.
Structure: Title, Executive Summary (bullet points), Introduction (context and background), Epidemiological features, Laboratory surveillance data, Risk assessment, Coordination with agencies, Central/local control measures, References.
Length: 3–6 pages.
5. Other Article Types
These categories include Editorial Note / Note from the field / Health Alert Reports / Guidelines for disease surveillance, investigation and control / Review article.
Authorship
- Editorial Note: Prepared by the Executive Editorial Board and the Academic Editorial Board.
- Note from the field / Health Alert Reports / Guidelines for disease surveillance, investigation and control / Review article: Prepared by personnel from the Division of Epidemiology or Department of Disease Control (DDC).
5.1 Notes from the Field
Brief reports on urgent or emerging events of public health importance.
Structure: Title, Executive Summary (bullet points), Background, Investigation and findings, Preliminary conclusions and control measures, Acknowledgements, References.
Length: 1–3 pages; <1 table/figure; >5 references.
5.2 Health Alert Reports
Reports providing timely information on health events to reduce public panic and limit disease spread.
Structure: Title, Executive Summary (bullet points), Background, Emerging trends (domestic and international), Relevant surveillance data, Public risk communication, Next steps, References.
Length: <4 pages.
5.3 Guidelines for disease surveillance, investigation and control
Technical articles describing surveillance mechanisms, case definitions, reporting procedures, outbreak investigation methods, specimen collection and submission, and related laboratory procedures.
Structure: Title, Background, Surveillance definitions, Case classifications, Reporting methods, Investigation protocols, Specimen handling, Diagnostic cost considerations, Appendices, References.
5.4 Review Articles Scholarly reviews summarizing existing knowledge from journals and books, domestic and international.
Structure: Abstract, Keywords, Introduction, Literature review, Commentary, Conclusion, References.
Length: <8 pages; <4 tables/figures/boxes; >10 references.



Submissions
As part of the submission process, authors are required to ensure that their manuscript complies with the following guidelines. Submissions that do not meet these requirements may be returned to the authors.
General Requirements:
- Originality: The manuscript must not have been previously published in any language or in a peer-reviewed journal, nor should it be under consideration for review elsewhere.
- Co-author Approval: All co-authors must acknowledge and approve the manuscript submission for publication.
- Data: If primary data is used, the data must not be older than five years from the completion of data collection.
- Online Submission: The Weekly Epidemiological Surveillance Report (WESR) exclusively accepts original articles submitted online via the ThaiJo system. Authors can submit their articles through the website. Click here
If the author wants to initiate a new submission or review a pending submission, please follow the link below: https://he05.tci-thaijo.org/index.php/WESR/about/submissions
Principal Author Confirmation:
- Submission of Original Manuscript Intent and Approval: The author can download the letter/memorandum of intent file to publish in WESR. This requires completing ‘Request for Publication Approval Form’ and the ‘WESR Author Submission Checklist’. The documents, along with the original manuscript file, must be attached and submitted to the ThaiJo system in PDF format.” Click
Review Process of Weekly Epidemiological Surveillance Report (WESR)
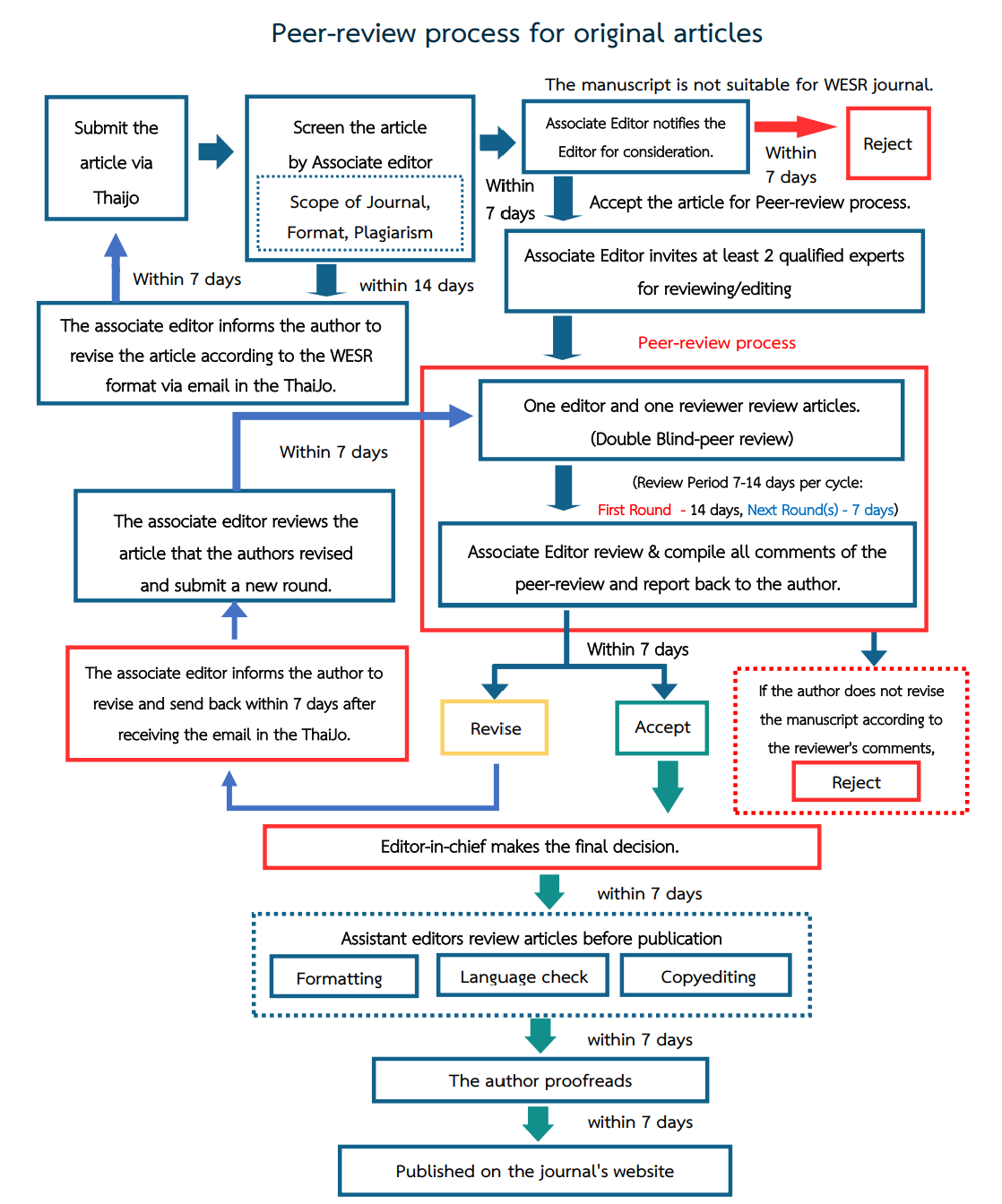
Author guidelines
Instructions for Authors
Principal Author Confirmation
- Submission of Original Manuscript Intent and Approval: The author can download the letter/memorandum of intent file to publish in WESR. This requires completing ‘Request for Publication Approval Form’ and the ‘WESR Author Submission Checklist’. The documents, along with the original manuscript file, must be attached and submitted to the ThaiJo system in PDF format.” Click
Author Information:
- Contact Details: The authors’ names, email addresses, telephone numbers, and institutional affiliations must be provided in accordance with the requirements outlined in the Author Guidelines (please refer to the WESR author guidelines).
Manuscript Content:
- Title, Abstract, and Keywords: The title, structured abstract, and keywords must be provided in both Thai and English, following the WESR author guidelines.
- Citations and References: All citations within the text and the reference list at the end of the manuscript should adhere to the Vancouver referencing style.
- Plagiarism: All non-original concepts must be properly cited to avoid plagiarism.
- Font and Format: The manuscript must be prepared using Microsoft Word, with the TH Sarabun New font Images, charts, and tables should be relevant to and enhance the content of the manuscript.
Specific format of original article
องค์ประกอบรายงาน
● Title
- The title should be concise and relevant to the context of the article. It must be presented in both Thai and English.
มีรายละเอียดแนวโน้มการพบโรคที่ต้องเฝ้าระวัง/
จำนวนผู้ป่วยและเสียชีวิตที่มีการระบาดในพท. ต่าง ๆ
มีข้อเสนอแนะและมาตรการในการควบคุมป้องกันโรคเบื้องต้น
ตารางนำเสนอในรูปแบบตารางภาษาอังกฤษ
< หรือ = 3 ตาราง รูปภาพ หรือกล่อง
● Author's name
- Please include full name and surname (without specifying titles) and place of work/affiliation in both Thai and English. In cases of multiple authors, list names in order of their contribution to the article. Use superscript numbers after the last names of all authors associated with the listed affiliations. Include email addresses and telephone numbers for contact.
ผลการสอบสวน การระบาดของโรค ปัจจัยเสี่ยง และการดำเนินการมาตรการป้องกันและควบคุมโรคที่ได้ทำไปแล้วหรือต้องทำต่อไปมีการประเมินความเสี่ยงในโรคที่มีการระบาดในช่วงนั้น ๆข่าวการระบาดของโรค/ภัยสุขภาพในต่างประเทศ (อาจมีหรือไม่มี)
<3 ตารางหรือรูปภาพเสริม (ไม่บังคับ)
● Abstract
- Please provide a concise summary of the important content, include only essential information and use clear and complete sentences. It should be in structured format cover the following: introduction and objectives, methods, results, discussion, and recommendations. Please avoid footnotes or references. The abstract should be written in both Thai (not exceeding 550 words) and English (not exceeding 450 words).
ประกอบด้วยหัวข้อ ไฮไลท์สรุปสั้น ๆ บทนำ (กล่าวถึงสถานการณ์โดย
รวม ใส่ความรู้เกี่ยวกับโรค หากเป็นโรคอุบัติใหม่หรือเป็นโรคหายาก) รายละเอียดลักษณะทางระบาดวิทยา ระบบเฝ้าระวังที่เกี่ยวข้อง การประสานงานร่วมกับหน่วยงานที่เกี่ยวข้องมาตรการดำเนินการควบ
คุมโรค/ภัยระดับส่วนกลาง/พื้นที่การสื่อสารความเสี่ยงสิ่งที่ต้องดำเนิน การต่อไป เอกสารอ้างอิง และความยาวของบทความไม่ควรเกิน 4 หน้า
● Content
- The content should use language that is clear, concise, and easily understandable. If using abbreviations, spell out the full word initially. Please minimize verbosity (e.g., replace phrases like “equal to” with “=”). When using scientific units, please write them out in full rather than using abbreviations.
ประกอบด้วยหัวข้อ ไฮไลท์สรุปสั้น ๆ เกริ่นนำ/ความเป็นมา แนวโน้มที่จะเกิดการะบาด/พบโรคในประเทศและต่างประเทศ ข้อมูลการเฝ้าระวังโรค/ภัยสุขภาพของประเทศที่เกี่ยวข้อง การสื่อสารความเสี่ยงต่อสาธารณะ ในพท./ภาพรวมประเทศ สิ่งที่ต้องดำเนินการต่อไป กิตติกรรมประกาศ เอกสารอ้างอิง และความยาวไม่ควรเกิน 4 หน้า
● Introduction
- The introduction part should give a background and significance of the topic including a literature review of related works and outline the study’s objectives.
ประกอบด้วยหัวข้อ บทนำ การดำเนินการสอบสวนเบื้องต้น สถานการณ์โรค/ภัยสุขภาพในพื้นที่ รายละเอียดผู้ป่วย/การระบาด ผลการตรวจทางห้องปฏิบัติการ/สิ่งแวดล้อม สรุปการสอบสวนเบื้องต้น มาตรการดำเนินการควบคุมโรค/ภัยสุขภาพ การประสานงานร่วมกับหน่วยงานที่เกี่ยวข้องในการควบคุมโรค การสื่อสารความเสี่ยงในระดับส่วนกลาง/พื้นที่ สิ่งที่ต้องดำเนินการต่อไป กิตติกรรมประกาศ เอกสารอ้างอิง และความยาวไม่ควรเกิน 4 หน้า
● Materials and Methods
- The section describes the research methods and data sources. It explains how data were collected, sampling methods, tools, and data analysis and statistics used.
ประกอบด้วยองค์ประกอบหลักและลำดับเนื้อเรื่องดังต่อไปนี้ บทคัดย่อ คำสำคัญ บทนำ วิธีการศึกษา ผลการศึกษา อภิปราย ข้อจำกัดในการ
ศึกษา มาตรการควบคุมและป้องกันโรค ข้อเสนอแนะ กิตติกรรมประกาศ และเอกสารอ้างอิง ความยาวของเรื่อง < หรือ = 14 หน้า < หรือ = 5 ตาราง รูปภาพ หรือกล่องการอ้างอิงขั้นต่ำที่จำเป็นเพื่อสนับสนุนคำแนะนำ < หรือ = 10 เชิงอรรถ
● Results
- It explains the results in detail, following with the study’s methods. The author should interpret the results or analyses.
ควรประกอบด้วยองค์ประกอบหลักและลำดับเนื้อเรื่องดังต่อไปนี้ บทคัดย่อ บทนำ วิธีการศึกษา วัสดุและวิธีการศึกษา ผลการศึกษา อภิปราย ข้อจำกัดในการศึกษา สรุป ข้อเสนอแนะ กิตติกรรมประกาศ เอกสารอ้างอิง ความยาวของเรื่อง < หรือ = 14 หน้า < หรือ = 5 ตาราง รูปภาพ หรือกล่อง < หรือ = 10 เชิงอรรถ
● Summary and discussion
- The author analyzes the study’s results and whether they align with the previous knowledge. Please reference relevant theories or studies.
ควรประกอบด้วยองค์ประกอบหลักและตามด้วยลำดับเนื้อเรื่อง
ดังต่อไป บทคัดย่อ คำสำคัญ บทนำ วิธีการศึกษา ผลการศึกษา อภิปราย ข้อจำกัดในการศึกษา สรุป ข้อเสนอแนะ กิตติกรรมประกาศ และเอกสารอ้างอิงความยาวของเรื่อง < หรือ = 14 หน้า < หรือ = 4 ตาราง รูปภาพ หรือกล่อง < หรือ = 10 การอ้างอิง
● Recommendation
- This section should provide practical guidance to readers and demonstrate how the research can have a significant impact on disease control or public health. Recommendations should be supported by the study results and discussion, and clearly specify who/which organization should take what action, with whom/which entity, and when.
ประกอบด้วยองค์ประกอบหลักและลำดับเนื้อเรื่องดังต่อไปนี้ บทคัดย่อ คำสำคัญ บทนำ วิธีการศึกษา ผลการศึกษา สรุปและวิจารณ์ ข้อจำกัดในการศึกษา มาตรการควบคุมและป้องกันโรค ข้อเสนอแนะ กิตติกรรมประกาศ และเอกสารอ้างอิง
ความยาวของเรื่อง < หรือ = 14 หน้า
● Conclusion
- Summarize the study and provide recommendations aligned with the objectives stated in the manuscript.
ประกอบด้วยบทคัดย่อ บทนำ ความรู้หรือข้อมูลเกี่ยวกับเรื่องนั้น บทวิจารณ์ และสรุปผลจากความคิดเห็นของผู้เขียน เอกสารอ้างอิงควรเป็นปัจจุบัน ความยาวของเรื่อง < หรือ = 8 หน้า < หรือ = 4 ตาราง รูปภาพหรือกล่อง < หรือ = 10 การอ้างอิง
● Limitations
- Characteristics of the data or analysis that may affect the accuracy or validity of the results. It is written in paragraph form in sequence. For example, ‘1st, 2nd, and 3rd’, ending with ‘For the final constraint’ specifies how each constraint may affect the results.
ประกอบด้วย สถานการณ์โรค ข้อมูลคนไข้ บันทึกเวชกรรม (Clinic note) ลักษณะเวชกรรม (Case description) การดำเนินโรค (Clinic course) สรุปกรณีศึกษา วิจารณ์หรือข้อสังเกต การยินยอมอนุญาตของคนไข้ (informed consent) และเอกสารอ้างอิง ความยาวของเรื่อง < หรือ = 10 หน้า < หรือ = 4 ตาราง รูปภาพ หรือ
กล่อง < หรือ = 10 การอ้างอิง
● Acknowledgements
- Acknowledge individuals or organizations that supported the study. Persons named here must not appear on the author list.
● Ethical Considerations
- All research articles must include a statement of ethical approval, specifying the name of the ethics committee, protocol number, and approval date.
● Conflicts of Interest
- Authors must disclose any conflicts of interest related to the study. If none exist, state: “The authors declare no conflicts of interest.”
● Funding Support
- Authors should specify the source of financial support (if any), including the name(s) of the funding organization(s) and the purpose of the funding.
● Declaration of Generative AI and AI-Assisted Technologies in the Writing Process
- If generative AI or AI-assisted tools were used to draft, revise, or improve the manuscript, this must be disclosed using the following statement (with bracketed text replaced as appropriate):
“During the preparation of this work, the author(s) used [TOOL NAME] to [PURPOSE, e.g., ‘enhance clarity’ or ‘check grammar’].
The content produced was reviewed and edited by the author(s), who take full responsibility for the final version.”
The use of standard spelling/grammar checkers or reference management tools does not require disclosure. No AI system should be listed as an author or co-author. If no such technologies were used, this section may be omitted.
● Figures and tables:
- The figure and table titles provide detailed descriptions that match the content. Place the table title above the table, and the figure title below the figure, whether it is an image, graph, chart, or map. Number them all as ‘Figure’ or ‘Table.’ If it’s a graph, include labels for the X and Y axes in the graph description only, and it should be editable. Use the TH Sarabun New font for the text in the figure or table, with a minimum font size of 12.
● Illustrations, diagrams, or pictures
- All would be in black color. If color is necessary, employ different patterns for clarity. Photos should be included in the presentation file or provided as full-color postcards with a separate description. Do not write directly on the images.
● Decimal Usage
- For decimal representation within the manuscript, one or two decimal places are acceptable. Please ensure a consistent decimal format is used throughout the entire document.
● Keywords
- Please select keywords that reflect the article’s content. Please use 3-5 words that capture the main idea of the article and place keywords at the end of the abstract in both Thai and English to facilitate content searching and access.
● References
- Please follow Vancouver-style references, using English for all references. If the original reference is in Thai, authors must provide an English translation and indicate “(in Thai)” at the end of the reference. The author is responsible for the accuracy of all referenced documents. Each reference within the article should be numbered consecutively, starting from number 1 and continuing in sequence throughout the text. When citing an article more than once, use the same reference number. For foreign journal references, use the initials as per the Index Medicus guidelines. Any citation errors may result in delays in the submission process, as additional details may be required from the author to ensure compliance.
● Referencing Format (please notice the punctuation marks in the examples)
- Journal Articles
Numeric order. Authors’ Names (Surnames and Initials). Title of the Article. Abbreviated Journal Name. Year of Publication; Journal Volume: First page – Last page. In case there are more than 6 authors, the first 6 authors are listed, followed by et al.
Example: Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009; 62: 1006-12.
- Textbooks and handbooks divided into 2 types
- Book
Numeric order. Authors’ Names (Surnames and Initials). Book Title. Edition. City of Publication: Publisher; Year of Publication.
Example: Toman K. Tuberculosis case-finding and chemo-therapy. Geneva: World Health Organization; 1979.
- Book chapters chapter in an edited book
Numeric order. Authors’ Names. Chapter Title. In; (Editors’ Names), Editor. Book Title. Edition. City of Publication: Publisher; Year of Publication. First page – Last page
Example: Becker MH, Maiman LA. The Health Belief Model and Sick Role Behavior. In: Becker MH, editor. The health belief model and personal health behavior. New Jersey: Charles B. Slack, Inc; 1974. pp. 82–92.
- Conference proceeding
Numeric order. Editors’ Names, Editor(s). Title. Conference Name; Conference Date; Conference Venue. City of Publication: Publisher; Year of Publication.
Example: Kimura J, Shibasaki H, editors. Recent advances in clinical neurophysiology. Proceedings of the 10th International Congress of EMG and Clinical Neurophysiology; 1995 Oct 15-19; Kyoto, Japan. Amsterdam: Elsevier; 1996.
- Conference article
Numeric order. Author’s Name. Title. In: editor’s name, editor. Meeting name; Meeting date; Meeting place, Meeting City. City of Publication: Year of publication. p. First page-last page.
Example: Bengtsson S, Solheim BG. Enforcement of data protection, privacy and security in medical informatics. In: Lun KC, Degoulet P, Piemme TE, Rienhoff O, editors. MEDINFO 92. Proceedings of the 7th World Congress on Medical Informatics; 1992 Sep 6-10; Geneva, Switzerland. Amsterdam: North-Holland; 1992. p. 1561-5.
- Thesis
Numeric order. Author’s Name. Title [Thesis Type/ Degree]. City of Publication: University; Year of Graduation. Number of pages.
Example: Sansiritaweesook G. Development of a surveillance system to prevent drowning based on the participation of communities at Ubon Ratchathani Province [dissertation]. Khon Kaen: Khon Kaen University; 2012. 391 p. (in Thai)
- Electronic documents
- Electronic Journal: Numeric order. Author’s Name. Title. Journal Name [Media type]. Publication Year [Retrieved/ Cited Date]; volume: First page – Last page. Access/ Available from: https://………………
Example: Arora M, Chauhan K, John S, Mukhopadhyay A. Multi–sectoral action for addressing social determinants of noncommunicable diseases and mainstreaming health promotion in national health programmes in India. Indian J Community Med [Internet]. 2011 [cited 2022 Dec 25];36:S43–9. Available from: https://pubmed.ncbi.nlm.nih.gov/22 628911/
- Electronic Books or Articles: Numeric order. Author’s Name. Title [Type of media]. Printed city. Publisher; Publication Year [Retrieved / cited Year, Month, Date]. Number of Pages. Source / Available from: https: // …………
Example: Merlis M, Gould D, Mahato B. Rising out-of-pocket spending for medical care: a growing strain on family budgets [Internet]. New York: Commonwealth Fund; 2006 Feb [cited 2006 Oct 2]. 23 p. Available from: https://www.cmwf.org/usr_doc/Merlis_risingoopspending_887.pdf
- Other
Government agencies or national and international organizations support the production and dissemination.
The name of those organizations should be placed in the same position as that of the publisher. In case the nationality is not included in the organization’s name, two English letters designating the country code according to the ISO 3166 standard should be placed in parenthesis immediately after the organization’s name, for example:
- Department of Disease Control (TH)
- Department of Health (AU)
- Centers for Disease Control and Prevention (US)
- Office of the Permanent Secretary, Ministry of Public Health (TH)
Copyright Notice
Copyright Notice
1. The content and information in articles published with WESR are the opinions and responsibilities of the authors of the articles directly, which the journal editorial team does not need to agree with or share any responsibility.
2. Articles, information, content, images, etc. published in WESR are considered to be copyrighted by academic journals. If any person or entity wants to publish all or part of it, any part of it shall be disseminated. Please refer to the article.
Privacy Policy
Personal Data Protection Act, B.E. 2567 (2024) Weekly Epidemiological Surveillance Report (WESR) journal
The Weekly Epidemiological Surveillance Report (WESR) journal is important in compliance with the Personal Data Protection Act B.E. 2562, effective May 28, 2019, and in accordance with the data protection laws to ensure that data owners trust that the journal will take care of their personal data and implement appropriate security measures.
- Definition
‘Journal’ refers to the Weekly Epidemiological Surveillance Report (WESR).
‘Person’ refers to an ordinary individual.
‘Personal data’ refers to information about a person that enables the identification of that individual, whether directly or indirectly, but does not include data about deceased individuals such as name, surname, address, telephone number, email address, IP address, cookie ID, log files, etc. However, the following information is not considered personal data: business contact information that does not identify an individual, such as company name, company address, company registration number, work phone number, work email address, anonymous data or pseudonymous data that has been processed to prevent identification, and deceased individuals’ data.
‘Data controller’ refers to an individual or legal entity with the authority to make decisions about the collection, use, or disclosure of personal data.
‘Data processor’ refers to an individual or legal entity that processes personal data on behalf of, or under the authority of, the data controller. However, individuals or legal entities performing such activities are not data controllers.
‘Processing’ refers to the collection, use, or disclosure of personal data.
- Sources of personal data collected by the journal
The journal collects or obtains various types of personal data from the following sources:
2.1 The journal collects personal data directly from data subjects via various service channels such as registration, survey completion, photography, and videography at conferences or training events, or when data subjects communicate with the journal in person at its premises or through other controlled communication channels managed by the journal.
2.2 The journal collects data from data subjects who access websites, products, or other services under contracts or missions, such as tracking usage behaviors of websites, products, or services through the use of cookies or from software on the data subject’s devices.
2.3 The journal collects personal data from sources other than the data subjects themselves, where such sources are authorized, justified by law, or have obtained consent from the data subjects to disclose the information to the journal. For example, linking digital services of government agencies to provide public services efficiently to the data subjects themselves, receiving personal data from other government agencies as part of the journal’s mission to facilitate data exchange centers to support the operations of government agencies in providing services to the public through digital systems, as well as fulfilling contractual obligations that may involve exchanging personal data with contracting parties.
Furthermore, this also includes cases where you act as the provider of personal data belonging to an outsider to the journal. Therefore, you are responsible for informing the individuals concerned about the details according to this policy or the announcements of the product or service, as appropriate, and obtaining consent from those individuals if required to disclose information to the journal. In cases where the data subjects refuse to provide necessary information for the journal’s service, it may result in the journal being unable to provide the service to those data subjects in whole or in part.
- Processing of personal data
(1) Personal data collection
The journal will collect personal data to the extent necessary and limited, depending on the type of service used by the owner of personal data or providing personal data to the journal. For example, registering for participation in activities, registering for various services, registering for journal evaluation, etc., whether directly through the journal or through the journal’s information system, which will collect personal data only as necessary.
(2) Personal data usage
The journal will use personal data according to the purposes provided by the data subjects to the journal, utilizing it appropriately and implementing security measures to ensure confidentiality and control access to personal data.
(3) Disclosure of personal data
The journal may disclose your personal data to certain categories of individuals and channels, as follows:
- Your affiliated organization: For instance, in cases where your organization requests that the journal send reports of conference attendees who are personnel within the organization.
- Government officials, authorized agencies, or other individuals for lawful enforcement, official orders, court orders, etc.
- Network agencies, partners, service providers, or relevant individuals necessary for the services provided by the journal that are related to your data, such as database service providers, document delivery services, website developers, etc.
- Public announcements, such as information searches for published articles, announcements of conference attendee lists, images, video clips related to the conference activities, promotional advertisements featuring conference attendees or speakers appearing in part of the media, through the journal’s website, tci-thailand.org, and announcements via the journal’s social media channels, etc.
However, in cases where the disclosure of your personal data requires your prior consent, the journal will proceed to request consent in accordance with the terms and conditions of relevant laws.
- Purposes of processing personal data
The journal utilizes methods of collecting, using, and disclosing personal data in a lawful and fair manner, storing personal data only as necessary for providing services, publicity, or disseminating various information, as well as surveying the opinions of the data subjects regarding the journal’s operations or activities, solely for the purposes of the journal’s operations or as required by law. In the event of any changes in purposes, the journal will inform the data subjects and record additional details as evidence while adhering to data protection laws.
- Duration of personal data retention
The journal will retain personal data only for as long as necessary for processing purposes. Once the retention period has elapsed or there is no longer a necessity for further processing, the journal will proceed to delete, destroy, or anonymize the personal data to render it unidentifiable to the data subject.
- Rights of the data subject
The consent given by the owner of personal data to the Journal for the collection, use, and disclosure of personal data remains valid until the data subject withdraws their consent in writing. The data subject has the right to withdraw consent or suspend the use or disclosure of personal data for any or all activities of the journal. This can be done by submitting a written request to the journal, either in hard copy or via email to wesr@ddc.mail.go.th
In addition to the aforementioned rights, the data subject also has the right to protect your fundamental data, as follows:
(1) Right to withdraw consent: The data subject has the right to withdraw consent for the processing of personal data provided to the journal at any time during the period in which their personal data is held by the journal.
(2) Right of access: The data subject has the right to access their personal data and request the journal to provide a copy of such personal data. Including requesting the journal disclose the source of personal data obtained by the journal without the data subject’s consent.
(3) Right to rectification: The data subject has the right to request the journal correct inaccurate personal data or supplement incomplete personal data.
(4) Right to erasure: The data subject has the right to request the journal delete their personal data for certain reasons.
(5) Right to restriction of processing: The data subject has the right to request the suspension of processing of their personal data for certain reasons.
(6) Right to data portability: The data subject has the right to request that their personal data, which they have provided to the journal, be transferred to another data controller or to themselves for certain reasons.
(7) Right to object: The data subject has the right to object to the processing of their personal data for certain reasons. The journal respects the decision to withdraw consent by the data subject; however, it should be noted that there may be limitations on the right to withdraw consent under law or contract benefiting the data subject. The withdrawal of consent has no effect on the processing, use, or disclosure of personal data to which the data subject previously consented.
- Security measures for personal data protection
The journal implements appropriate security measures to prevent unauthorized access, use, alteration, modification, or disclosure of personal data. Additionally, the journal establishes internal practices within the organization to define the rights of access or use of personal data by data owners, ensuring the confidentiality and security of data. Periodic reviews of these measures are conducted for appropriateness.
- Website usage data
8.1 While you use the WESR website, the journal will track and record your visits to each page of the website in server log files (“Usage Data”). This usage data may be used by WESR for statistical purposes to understand website usage trends and to improve the structure and presentation of content. The usage data collected by the journal (using HTTP cookies) includes URLs visited, browser and device characteristics, IP address, operating system, and the date and time of website visits. When the data subject visits the website again, the website can recognize that it is a returning user and adjust settings as specified by the data subject until the data subject deletes or refuses cookies. The data subject may choose to accept or decline cookies. In the event of declining or deleting cookies, the website may not be able to provide services or display content correctly.
8.2 The WESR website may use Google Analytics to help collect data on how the WESR website is visited. The journal will use this data to compile reports and help us improve the website. The cookie tools will collect data in an anonymous form, including the number of visitors to the website, how individuals arrived at the website, from which browser or web page, and which web pages those individuals visited while on the website.
- Updating the Personal Data Protection Policy
The journal may update or amend the Personal Data Protection Policy without prior notice to the data subjects. This is to ensure appropriateness and efficiency in service provision. Therefore, the journal recommends that data subjects read the Personal Data Protection Policy every time they visit or use services from the journal or its website.
- Compliance with the Personal Data Protection Policy and Contacting the Journal
If data subjects have any questions or suggestions regarding this Personal Data Protection Policy or its implementation, the Journal welcomes inquiries and feedback for the continuous improvement of data protection and services. Data subjects can contact the journal at wesr@ddc.mail.go.th or at the address provided below.
Type of articles
The Weekly Epidemiological Surveillance Report (WESR) classifies published articles into five main categories.
- Original articles
- Outbreak investigation
- Public health program evaluation and surveillance evaluation
- Epidemiological study
- Case report
- Disease prevention and control-related research study
- Weekly summary report of disease outbreaks/health threat verifications
- Summary report of epidemiological surveillance activities
- Disease/Health Threat Situational Report
- Other Article Types: Editorial Note/Note from the field/Health Alert Reports/Guidelines for disease surveillance, investigation and control/Review article
WESR is published on a basis comprising 2-3 original articles, weekly summary report of disease outbreak/health threat verifications published 4-5 articles, summary report of epidemiological surveillance activities published 2-3 articles, disease/health threat situational report published 1-2 articles, and other article types are available on a case-by-case basis.
Types of articles published in WESR
1. Original articles
1.1 Epidemiological Investigations:
Reports of outbreak or public health threat investigations, including causative factors, sources, transmission, and patterns of spread.
Structure: Abstract, Keywords, Introduction, Methods, Ethical Considerations, Results, Discussion, Study Limitations, Control and Prevention Measures, Recommendations, Conclusion, Acknowledgements, Funding, Conflicts of Interest, AI Declaration, References.
Length: <14 pages; <4 tables/figures/boxes; >10 references.
1.2 Public Health Program or Surveillance Evaluation: Assessment of public health programs or surveillance systems related to communicable, noncommunicable, and occupational diseases.
Structure: Abstract, Keywords, Introduction, Methods, Ethical Considerations, Results, Discussion, Study Limitations, Recommendations, Conclusion, Acknowledgements, Funding, Conflicts of Interest, AI Declaration, References.
Length: <14 pages; <4 tables/figures/boxes; >10 references.
1.3 Epidemiological Studies:
Analysis of surveillance or statistical data at national or regional levels, describing person, place, and time distribution, with recommendations or policy implications.
Structure: Abstract, Keywords, Introduction, Methods, Ethical Considerations, Results, Discussion, Study Limitations, Recommendations, Conclusion, Acknowledgements, Funding, Conflicts of Interest, AI Declaration, References.
Length: <14 pages; <4 tables/figures/boxes; >10 references.
1.4 Case Reports: Detailed reports of rare or novel cases or syndromes.
Structure: Case background, Patient information, Clinical notes, Case description, Clinical course, Case summary, Discussion/Observations, Informed Consent, References.
Length: <10 pages; <4 tables/figures; >10 references.
1.5 Research Studies in Disease Prevention and Control:
Research addressing questions related to disease prevention, control measures, policy, or law, with ethical considerations.
Structure: Abstract, Introduction, Methods (including materials), Ethical Considerations, Results, Discussion, Study Limitations, Conclusion, Recommendations, Acknowledgements, Funding, Conflicts of Interest, AI Declaration, References.
Length: <14 pages; <5 tables/figures/boxes; >10 references.
2. Weekly Outbreak Verification Reports
Prepared by the WATCH team, summarizing weekly outbreak events from provincial health offices, regional disease control centers, Bangkok Metropolitan Administration, and other relevant agencies.
Content: Outbreak investigation details, risk factors, prevention and control measures taken or required, risk assessment, international outbreak reports (optional), supporting tables/figures (optional).
Length: <10 pages.
3. Epidemiological Surveillance Reports
Compiled by the Bureau of Epidemiology, presenting national-level patient and mortality data from notifiable disease surveillance systems.
Content: Case counts and deaths by area, trends, reporting coverage, and summary tables.




4. Disease/Health Threat Situation Reports
Prepared by staff of the Bureau of Epidemiology or Department of Disease Control, summarizing national or international disease situations.
Structure: Title, Executive Summary (bullet points), Introduction (context and background), Epidemiological features, Laboratory surveillance data, Risk assessment, Coordination with agencies, Central/local control measures, References.
Length: 3–6 pages.
5. Other Article Types
These categories include Editorial Note / Note from the field / Health Alert Reports / Guidelines for disease surveillance, investigation and control / Review article.
Authorship
- Editorial Note: Prepared by the Executive Editorial Board and the Academic Editorial Board.
- Note from the field / Health Alert Reports / Guidelines for disease surveillance, investigation and control / Review article: Prepared by personnel from the Division of Epidemiology or Department of Disease Control (DDC).
5.1 Notes from the Field
Brief reports on urgent or emerging events of public health importance.
Structure: Title, Executive Summary (bullet points), Background, Investigation and findings, Preliminary conclusions and control measures, Acknowledgements, References.
Length: 1–3 pages; <1 table/figure; >5 references.
5.2 Health Alert Reports
Reports providing timely information on health events to reduce public panic and limit disease spread.
Structure: Title, Executive Summary (bullet points), Background, Emerging trends (domestic and international), Relevant surveillance data, Public risk communication, Next steps, References.
Length: <4 pages.
5.3 Guidelines for disease surveillance, investigation and control
Technical articles describing surveillance mechanisms, case definitions, reporting procedures, outbreak investigation methods, specimen collection and submission, and related laboratory procedures.
Structure: Title, Background, Surveillance definitions, Case classifications, Reporting methods, Investigation protocols, Specimen handling, Diagnostic cost considerations, Appendices, References.
5.4 Review Articles Scholarly reviews summarizing existing knowledge from journals and books, domestic and international.
Structure: Abstract, Keywords, Introduction, Literature review, Commentary, Conclusion, References.
Length: <8 pages; <4 tables/figures/boxes; >10 references.



Submissions
As part of the submission process, authors are required to ensure that their manuscript complies with the following guidelines. Submissions that do not meet these requirements may be returned to the authors.
General Requirements:
- Originality: The manuscript must not have been previously published in any language or in a peer-reviewed journal, nor should it be under consideration for review elsewhere.
- Co-author Approval: All co-authors must acknowledge and approve the manuscript submission for publication.
- Data: If primary data is used, the data must not be older than five years from the completion of data collection.
- Online Submission: The Weekly Epidemiological Surveillance Report (WESR) exclusively accepts original articles submitted online via the ThaiJo system. Authors can submit their articles through the website. Click here
If the author wants to initiate a new submission or review a pending submission, please follow the link below: https://he05.tci-thaijo.org/index.php/WESR/about/submissions
Principal Author Confirmation:
- Submission of Original Manuscript Intent and Approval: The author can download the letter/memorandum of intent file to publish in WESR. This requires completing ‘Request for Publication Approval Form’ and the ‘WESR Author Submission Checklist’. The documents, along with the original manuscript file, must be attached and submitted to the ThaiJo system in PDF format.” Click
Review Process of Weekly Epidemiological Surveillance Report (WESR)

Author guidelines
Instructions for Authors
Principal Author Confirmation
- Submission of Original Manuscript Intent and Approval: The author can download the letter/memorandum of intent file to publish in WESR. This requires completing ‘Request for Publication Approval Form’ and the ‘WESR Author Submission Checklist’. The documents, along with the original manuscript file, must be attached and submitted to the ThaiJo system in PDF format.” Click
Author Information:
- Contact Details: The authors’ names, email addresses, telephone numbers, and institutional affiliations must be provided in accordance with the requirements outlined in the Author Guidelines (please refer to the WESR author guidelines).
Manuscript Content:
- Title, Abstract, and Keywords: The title, structured abstract, and keywords must be provided in both Thai and English, following the WESR author guidelines.
- Citations and References: All citations within the text and the reference list at the end of the manuscript should adhere to the Vancouver referencing style.
- Plagiarism: All non-original concepts must be properly cited to avoid plagiarism.
- Font and Format: The manuscript must be prepared using Microsoft Word, with the TH Sarabun New font Images, charts, and tables should be relevant to and enhance the content of the manuscript.
Specific format of original article
องค์ประกอบรายงาน
● Title
- The title should be concise and relevant to the context of the article. It must be presented in both Thai and English.
มีรายละเอียดแนวโน้มการพบโรคที่ต้องเฝ้าระวัง/
จำนวนผู้ป่วยและเสียชีวิตที่มีการระบาดในพท. ต่าง ๆ
มีข้อเสนอแนะและมาตรการในการควบคุมป้องกันโรคเบื้องต้น
ตารางนำเสนอในรูปแบบตารางภาษาอังกฤษ
< หรือ = 3 ตาราง รูปภาพ หรือกล่อง
● Author’s name
- Please include full name and surname (without specifying titles) and place of work/affiliation in both Thai and English. In cases of multiple authors, list names in order of their contribution to the article. Use superscript numbers after the last names of all authors associated with the listed affiliations. Include email addresses and telephone numbers for contact.
ผลการสอบสวน การระบาดของโรค ปัจจัยเสี่ยง และการดำเนินการมาตรการป้องกันและควบคุมโรคที่ได้ทำไปแล้วหรือต้องทำต่อไปมีการประเมินความเสี่ยงในโรคที่มีการระบาดในช่วงนั้น ๆข่าวการระบาดของโรค/ภัยสุขภาพในต่างประเทศ (อาจมีหรือไม่มี)
<3 ตารางหรือรูปภาพเสริม (ไม่บังคับ)
● Abstract
- Please provide a concise summary of the important content, include only essential information and use clear and complete sentences. It should be in structured format cover the following: introduction and objectives, methods, results, discussion, and recommendations. Please avoid footnotes or references. The abstract should be written in both Thai (not exceeding 550 words) and English (not exceeding 450 words).
ประกอบด้วยหัวข้อ ไฮไลท์สรุปสั้น ๆ บทนำ (กล่าวถึงสถานการณ์โดย
รวม ใส่ความรู้เกี่ยวกับโรค หากเป็นโรคอุบัติใหม่หรือเป็นโรคหายาก) รายละเอียดลักษณะทางระบาดวิทยา ระบบเฝ้าระวังที่เกี่ยวข้อง การประสานงานร่วมกับหน่วยงานที่เกี่ยวข้องมาตรการดำเนินการควบ
คุมโรค/ภัยระดับส่วนกลาง/พื้นที่การสื่อสารความเสี่ยงสิ่งที่ต้องดำเนิน การต่อไป เอกสารอ้างอิง และความยาวของบทความไม่ควรเกิน 4 หน้า
● Content
- The content should use language that is clear, concise, and easily understandable. If using abbreviations, spell out the full word initially. Please minimize verbosity (e.g., replace phrases like “equal to” with “=”). When using scientific units, please write them out in full rather than using abbreviations.
ประกอบด้วยหัวข้อ ไฮไลท์สรุปสั้น ๆ เกริ่นนำ/ความเป็นมา แนวโน้มที่จะเกิดการะบาด/พบโรคในประเทศและต่างประเทศ ข้อมูลการเฝ้าระวังโรค/ภัยสุขภาพของประเทศที่เกี่ยวข้อง การสื่อสารความเสี่ยงต่อสาธารณะ ในพท./ภาพรวมประเทศ สิ่งที่ต้องดำเนินการต่อไป กิตติกรรมประกาศ เอกสารอ้างอิง และความยาวไม่ควรเกิน 4 หน้า
● Introduction
- The introduction part should give a background and significance of the topic including a literature review of related works and outline the study’s objectives.
ประกอบด้วยหัวข้อ บทนำ การดำเนินการสอบสวนเบื้องต้น สถานการณ์โรค/ภัยสุขภาพในพื้นที่ รายละเอียดผู้ป่วย/การระบาด ผลการตรวจทางห้องปฏิบัติการ/สิ่งแวดล้อม สรุปการสอบสวนเบื้องต้น มาตรการดำเนินการควบคุมโรค/ภัยสุขภาพ การประสานงานร่วมกับหน่วยงานที่เกี่ยวข้องในการควบคุมโรค การสื่อสารความเสี่ยงในระดับส่วนกลาง/พื้นที่ สิ่งที่ต้องดำเนินการต่อไป กิตติกรรมประกาศ เอกสารอ้างอิง และความยาวไม่ควรเกิน 4 หน้า
● Materials and Methods
- The section describes the research methods and data sources. It explains how data were collected, sampling methods, tools, and data analysis and statistics used.
ประกอบด้วยองค์ประกอบหลักและลำดับเนื้อเรื่องดังต่อไปนี้ บทคัดย่อ คำสำคัญ บทนำ วิธีการศึกษา ผลการศึกษา อภิปราย ข้อจำกัดในการ
ศึกษา มาตรการควบคุมและป้องกันโรค ข้อเสนอแนะ กิตติกรรมประกาศ และเอกสารอ้างอิง ความยาวของเรื่อง < หรือ = 14 หน้า < หรือ = 5 ตาราง รูปภาพ หรือกล่องการอ้างอิงขั้นต่ำที่จำเป็นเพื่อสนับสนุนคำแนะนำ < หรือ = 10 เชิงอรรถ
● Results
- It explains the results in detail, following with the study’s methods. The author should interpret the results or analyses.
ควรประกอบด้วยองค์ประกอบหลักและลำดับเนื้อเรื่องดังต่อไปนี้ บทคัดย่อ บทนำ วิธีการศึกษา วัสดุและวิธีการศึกษา ผลการศึกษา อภิปราย ข้อจำกัดในการศึกษา สรุป ข้อเสนอแนะ กิตติกรรมประกาศ เอกสารอ้างอิง ความยาวของเรื่อง < หรือ = 14 หน้า < หรือ = 5 ตาราง รูปภาพ หรือกล่อง < หรือ = 10 เชิงอรรถ
● Summary and discussion
- The author analyzes the study’s results and whether they align with the previous knowledge. Please reference relevant theories or studies.
ควรประกอบด้วยองค์ประกอบหลักและตามด้วยลำดับเนื้อเรื่อง
ดังต่อไป บทคัดย่อ คำสำคัญ บทนำ วิธีการศึกษา ผลการศึกษา อภิปราย ข้อจำกัดในการศึกษา สรุป ข้อเสนอแนะ กิตติกรรมประกาศ และเอกสารอ้างอิงความยาวของเรื่อง < หรือ = 14 หน้า < หรือ = 4 ตาราง รูปภาพ หรือกล่อง < หรือ = 10 การอ้างอิง
● Recommendation
- This section should provide practical guidance to readers and demonstrate how the research can have a significant impact on disease control or public health. Recommendations should be supported by the study results and discussion, and clearly specify who/which organization should take what action, with whom/which entity, and when.
ประกอบด้วยองค์ประกอบหลักและลำดับเนื้อเรื่องดังต่อไปนี้ บทคัดย่อ คำสำคัญ บทนำ วิธีการศึกษา ผลการศึกษา สรุปและวิจารณ์ ข้อจำกัดในการศึกษา มาตรการควบคุมและป้องกันโรค ข้อเสนอแนะ กิตติกรรมประกาศ และเอกสารอ้างอิง
ความยาวของเรื่อง < หรือ = 14 หน้า
● Conclusion
- Summarize the study and provide recommendations aligned with the objectives stated in the manuscript.
ประกอบด้วยบทคัดย่อ บทนำ ความรู้หรือข้อมูลเกี่ยวกับเรื่องนั้น บทวิจารณ์ และสรุปผลจากความคิดเห็นของผู้เขียน เอกสารอ้างอิงควรเป็นปัจจุบัน ความยาวของเรื่อง < หรือ = 8 หน้า < หรือ = 4 ตาราง รูปภาพหรือกล่อง < หรือ = 10 การอ้างอิง
● Limitations
- Characteristics of the data or analysis that may affect the accuracy or validity of the results. It is written in paragraph form in sequence. For example, ‘1st, 2nd, and 3rd’, ending with ‘For the final constraint’ specifies how each constraint may affect the results.
ประกอบด้วย สถานการณ์โรค ข้อมูลคนไข้ บันทึกเวชกรรม (Clinic note) ลักษณะเวชกรรม (Case description) การดำเนินโรค (Clinic course) สรุปกรณีศึกษา วิจารณ์หรือข้อสังเกต การยินยอมอนุญาตของคนไข้ (informed consent) และเอกสารอ้างอิง ความยาวของเรื่อง < หรือ = 10 หน้า < หรือ = 4 ตาราง รูปภาพ หรือ
กล่อง < หรือ = 10 การอ้างอิง
● Acknowledgements
- Acknowledge individuals or organizations that supported the study. Persons named here must not appear on the author list.
● Ethical Considerations
- All research articles must include a statement of ethical approval, specifying the name of the ethics committee, protocol number, and approval date.
● Conflicts of Interest
- Authors must disclose any conflicts of interest related to the study. If none exist, state: “The authors declare no conflicts of interest.”
● Funding Support
- Authors should specify the source of financial support (if any), including the name(s) of the funding organization(s) and the purpose of the funding.
● Declaration of Generative AI and AI-Assisted Technologies in the Writing Process
- If generative AI or AI-assisted tools were used to draft, revise, or improve the manuscript, this must be disclosed using the following statement (with bracketed text replaced as appropriate):
“During the preparation of this work, the author(s) used [TOOL NAME] to [PURPOSE, e.g., ‘enhance clarity’ or ‘check grammar’].
The content produced was reviewed and edited by the author(s), who take full responsibility for the final version.”
The use of standard spelling/grammar checkers or reference management tools does not require disclosure. No AI system should be listed as an author or co-author. If no such technologies were used, this section may be omitted.
● Figures and tables:
- The figure and table titles provide detailed descriptions that match the content. Place the table title above the table, and the figure title below the figure, whether it is an image, graph, chart, or map. Number them all as ‘Figure’ or ‘Table.’ If it’s a graph, include labels for the X and Y axes in the graph description only, and it should be editable. Use the TH Sarabun New font for the text in the figure or table, with a minimum font size of 12.
● Illustrations, diagrams, or pictures
- All would be in black color. If color is necessary, employ different patterns for clarity. Photos should be included in the presentation file or provided as full-color postcards with a separate description. Do not write directly on the images.
● Decimal Usage
- For decimal representation within the manuscript, one or two decimal places are acceptable. Please ensure a consistent decimal format is used throughout the entire document.
● Keywords
- Please select keywords that reflect the article’s content. Please use 3-5 words that capture the main idea of the article and place keywords at the end of the abstract in both Thai and English to facilitate content searching and access.
● References
- Please follow Vancouver-style references, using English for all references. If the original reference is in Thai, authors must provide an English translation and indicate “(in Thai)” at the end of the reference. The author is responsible for the accuracy of all referenced documents. Each reference within the article should be numbered consecutively, starting from number 1 and continuing in sequence throughout the text. When citing an article more than once, use the same reference number. For foreign journal references, use the initials as per the Index Medicus guidelines. Any citation errors may result in delays in the submission process, as additional details may be required from the author to ensure compliance.
● Referencing Format (please notice the punctuation marks in the examples)
- Journal Articles
Numeric order. Authors’ Names (Surnames and Initials). Title of the Article. Abbreviated Journal Name. Year of Publication; Journal Volume: First page – Last page. In case there are more than 6 authors, the first 6 authors are listed, followed by et al.
Example: Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009; 62: 1006-12.
- Textbooks and handbooks divided into 2 types
- Book
Numeric order. Authors’ Names (Surnames and Initials). Book Title. Edition. City of Publication: Publisher; Year of Publication.
Example: Toman K. Tuberculosis case-finding and chemo-therapy. Geneva: World Health Organization; 1979.
- Book chapters chapter in an edited book
Numeric order. Authors’ Names. Chapter Title. In; (Editors’ Names), Editor. Book Title. Edition. City of Publication: Publisher; Year of Publication. First page – Last page
Example: Becker MH, Maiman LA. The Health Belief Model and Sick Role Behavior. In: Becker MH, editor. The health belief model and personal health behavior. New Jersey: Charles B. Slack, Inc; 1974. pp. 82–92.
- Conference proceeding
Numeric order. Editors’ Names, Editor(s). Title. Conference Name; Conference Date; Conference Venue. City of Publication: Publisher; Year of Publication.
Example: Kimura J, Shibasaki H, editors. Recent advances in clinical neurophysiology. Proceedings of the 10th International Congress of EMG and Clinical Neurophysiology; 1995 Oct 15-19; Kyoto, Japan. Amsterdam: Elsevier; 1996.
- Conference article
Numeric order. Author’s Name. Title. In: editor’s name, editor. Meeting name; Meeting date; Meeting place, Meeting City. City of Publication: Year of publication. p. First page-last page.
Example: Bengtsson S, Solheim BG. Enforcement of data protection, privacy and security in medical informatics. In: Lun KC, Degoulet P, Piemme TE, Rienhoff O, editors. MEDINFO 92. Proceedings of the 7th World Congress on Medical Informatics; 1992 Sep 6-10; Geneva, Switzerland. Amsterdam: North-Holland; 1992. p. 1561-5.
- Thesis
Numeric order. Author’s Name. Title [Thesis Type/ Degree]. City of Publication: University; Year of Graduation. Number of pages.
Example: Sansiritaweesook G. Development of a surveillance system to prevent drowning based on the participation of communities at Ubon Ratchathani Province [dissertation]. Khon Kaen: Khon Kaen University; 2012. 391 p. (in Thai)
- Electronic documents
- Electronic Journal: Numeric order. Author’s Name. Title. Journal Name [Media type]. Publication Year [Retrieved/ Cited Date]; volume: First page – Last page. Access/ Available from: https://………………
Example: Arora M, Chauhan K, John S, Mukhopadhyay A. Multi–sectoral action for addressing social determinants of noncommunicable diseases and mainstreaming health promotion in national health programmes in India. Indian J Community Med [Internet]. 2011 [cited 2022 Dec 25];36:S43–9. Available from: https://pubmed.ncbi.nlm.nih.gov/22 628911/
- Electronic Books or Articles: Numeric order. Author’s Name. Title [Type of media]. Printed city. Publisher; Publication Year [Retrieved / cited Year, Month, Date]. Number of Pages. Source / Available from: https: // …………
Example: Merlis M, Gould D, Mahato B. Rising out-of-pocket spending for medical care: a growing strain on family budgets [Internet]. New York: Commonwealth Fund; 2006 Feb [cited 2006 Oct 2]. 23 p. Available from: https://www.cmwf.org/usr_doc/Merlis_risingoopspending_887.pdf
- Other
Government agencies or national and international organizations support the production and dissemination.
The name of those organizations should be placed in the same position as that of the publisher. In case the nationality is not included in the organization’s name, two English letters designating the country code according to the ISO 3166 standard should be placed in parenthesis immediately after the organization’s name, for example:
- Department of Disease Control (TH)
- Department of Health (AU)
- Centers for Disease Control and Prevention (US)
- Office of the Permanent Secretary, Ministry of Public Health (TH)
Copyright Notice
Copyright Notice
1. The content and information in articles published with WESR are the opinions and responsibilities of the authors of the articles directly, which the journal editorial team does not need to agree with or share any responsibility.
2. Articles, information, content, images, etc. published in WESR are considered to be copyrighted by academic journals. If any person or entity wants to publish all or part of it, any part of it shall be disseminated. Please refer to the article.
Privacy Policy
Personal Data Protection Act, B.E. 2567 (2024) Weekly Epidemiological Surveillance Report (WESR) journal
The Weekly Epidemiological Surveillance Report (WESR) journal is important in compliance with the Personal Data Protection Act B.E. 2562, effective May 28, 2019, and in accordance with the data protection laws to ensure that data owners trust that the journal will take care of their personal data and implement appropriate security measures.
- Definition
‘Journal’ refers to the Weekly Epidemiological Surveillance Report (WESR).
‘Person’ refers to an ordinary individual.
‘Personal data’ refers to information about a person that enables the identification of that individual, whether directly or indirectly, but does not include data about deceased individuals such as name, surname, address, telephone number, email address, IP address, cookie ID, log files, etc. However, the following information is not considered personal data: business contact information that does not identify an individual, such as company name, company address, company registration number, work phone number, work email address, anonymous data or pseudonymous data that has been processed to prevent identification, and deceased individuals’ data.
‘Data controller’ refers to an individual or legal entity with the authority to make decisions about the collection, use, or disclosure of personal data.
‘Data processor’ refers to an individual or legal entity that processes personal data on behalf of, or under the authority of, the data controller. However, individuals or legal entities performing such activities are not data controllers.
‘Processing’ refers to the collection, use, or disclosure of personal data.
- Sources of personal data collected by the journal
The journal collects or obtains various types of personal data from the following sources:
2.1 The journal collects personal data directly from data subjects via various service channels such as registration, survey completion, photography, and videography at conferences or training events, or when data subjects communicate with the journal in person at its premises or through other controlled communication channels managed by the journal.
2.2 The journal collects data from data subjects who access websites, products, or other services under contracts or missions, such as tracking usage behaviors of websites, products, or services through the use of cookies or from software on the data subject’s devices.
2.3 The journal collects personal data from sources other than the data subjects themselves, where such sources are authorized, justified by law, or have obtained consent from the data subjects to disclose the information to the journal. For example, linking digital services of government agencies to provide public services efficiently to the data subjects themselves, receiving personal data from other government agencies as part of the journal’s mission to facilitate data exchange centers to support the operations of government agencies in providing services to the public through digital systems, as well as fulfilling contractual obligations that may involve exchanging personal data with contracting parties.
Furthermore, this also includes cases where you act as the provider of personal data belonging to an outsider to the journal. Therefore, you are responsible for informing the individuals concerned about the details according to this policy or the announcements of the product or service, as appropriate, and obtaining consent from those individuals if required to disclose information to the journal. In cases where the data subjects refuse to provide necessary information for the journal’s service, it may result in the journal being unable to provide the service to those data subjects in whole or in part.
- Processing of personal data
(1) Personal data collection
The journal will collect personal data to the extent necessary and limited, depending on the type of service used by the owner of personal data or providing personal data to the journal. For example, registering for participation in activities, registering for various services, registering for journal evaluation, etc., whether directly through the journal or through the journal’s information system, which will collect personal data only as necessary.
(2) Personal data usage
The journal will use personal data according to the purposes provided by the data subjects to the journal, utilizing it appropriately and implementing security measures to ensure confidentiality and control access to personal data.
(3) Disclosure of personal data
The journal may disclose your personal data to certain categories of individuals and channels, as follows:
- Your affiliated organization: For instance, in cases where your organization requests that the journal send reports of conference attendees who are personnel within the organization.
- Government officials, authorized agencies, or other individuals for lawful enforcement, official orders, court orders, etc.
- Network agencies, partners, service providers, or relevant individuals necessary for the services provided by the journal that are related to your data, such as database service providers, document delivery services, website developers, etc.
- Public announcements, such as information searches for published articles, announcements of conference attendee lists, images, video clips related to the conference activities, promotional advertisements featuring conference attendees or speakers appearing in part of the media, through the journal’s website, tci-thailand.org, and announcements via the journal’s social media channels, etc.
However, in cases where the disclosure of your personal data requires your prior consent, the journal will proceed to request consent in accordance with the terms and conditions of relevant laws.
- Purposes of processing personal data
The journal utilizes methods of collecting, using, and disclosing personal data in a lawful and fair manner, storing personal data only as necessary for providing services, publicity, or disseminating various information, as well as surveying the opinions of the data subjects regarding the journal’s operations or activities, solely for the purposes of the journal’s operations or as required by law. In the event of any changes in purposes, the journal will inform the data subjects and record additional details as evidence while adhering to data protection laws.
- Duration of personal data retention
The journal will retain personal data only for as long as necessary for processing purposes. Once the retention period has elapsed or there is no longer a necessity for further processing, the journal will proceed to delete, destroy, or anonymize the personal data to render it unidentifiable to the data subject.
- Rights of the data subject
The consent given by the owner of personal data to the Journal for the collection, use, and disclosure of personal data remains valid until the data subject withdraws their consent in writing. The data subject has the right to withdraw consent or suspend the use or disclosure of personal data for any or all activities of the journal. This can be done by submitting a written request to the journal, either in hard copy or via email to wesr@ddc.mail.go.th
In addition to the aforementioned rights, the data subject also has the right to protect your fundamental data, as follows:
(1) Right to withdraw consent: The data subject has the right to withdraw consent for the processing of personal data provided to the journal at any time during the period in which their personal data is held by the journal.
(2) Right of access: The data subject has the right to access their personal data and request the journal to provide a copy of such personal data. Including requesting the journal disclose the source of personal data obtained by the journal without the data subject’s consent.
(3) Right to rectification: The data subject has the right to request the journal correct inaccurate personal data or supplement incomplete personal data.
(4) Right to erasure: The data subject has the right to request the journal delete their personal data for certain reasons.
(5) Right to restriction of processing: The data subject has the right to request the suspension of processing of their personal data for certain reasons.
(6) Right to data portability: The data subject has the right to request that their personal data, which they have provided to the journal, be transferred to another data controller or to themselves for certain reasons.
(7) Right to object: The data subject has the right to object to the processing of their personal data for certain reasons. The journal respects the decision to withdraw consent by the data subject; however, it should be noted that there may be limitations on the right to withdraw consent under law or contract benefiting the data subject. The withdrawal of consent has no effect on the processing, use, or disclosure of personal data to which the data subject previously consented.
- Security measures for personal data protection
The journal implements appropriate security measures to prevent unauthorized access, use, alteration, modification, or disclosure of personal data. Additionally, the journal establishes internal practices within the organization to define the rights of access or use of personal data by data owners, ensuring the confidentiality and security of data. Periodic reviews of these measures are conducted for appropriateness.
- Website usage data
8.1 While you use the WESR website, the journal will track and record your visits to each page of the website in server log files (“Usage Data”). This usage data may be used by WESR for statistical purposes to understand website usage trends and to improve the structure and presentation of content. The usage data collected by the journal (using HTTP cookies) includes URLs visited, browser and device characteristics, IP address, operating system, and the date and time of website visits. When the data subject visits the website again, the website can recognize that it is a returning user and adjust settings as specified by the data subject until the data subject deletes or refuses cookies. The data subject may choose to accept or decline cookies. In the event of declining or deleting cookies, the website may not be able to provide services or display content correctly.
8.2 The WESR website may use Google Analytics to help collect data on how the WESR website is visited. The journal will use this data to compile reports and help us improve the website. The cookie tools will collect data in an anonymous form, including the number of visitors to the website, how individuals arrived at the website, from which browser or web page, and which web pages those individuals visited while on the website.
- Updating the Personal Data Protection Policy
The journal may update or amend the Personal Data Protection Policy without prior notice to the data subjects. This is to ensure appropriateness and efficiency in service provision. Therefore, the journal recommends that data subjects read the Personal Data Protection Policy every time they visit or use services from the journal or its website.
- Compliance with the Personal Data Protection Policy and Contacting the Journal
If data subjects have any questions or suggestions regarding this Personal Data Protection Policy or its implementation, the Journal welcomes inquiries and feedback for the continuous improvement of data protection and services. Data subjects can contact the journal at wesr@ddc.mail.go.th or at the address provided below.
